Study design. TEAE, treatmentemergent adverse event. Download Scientific Diagram
PPT Clinical Safety of Fospropofol Disodium During Diagnostic and Therapeutic Procedures
An adverse reaction, the nature or severity of which is not consistent with the applicable product information (e.g., Investigator's Brochure for an unapproved investigational medicinal product). (See section III.C.) B. Serious Adverse Event or Adverse Drug Reaction During clinical investigations, adverse events may occur which, if suspected to be
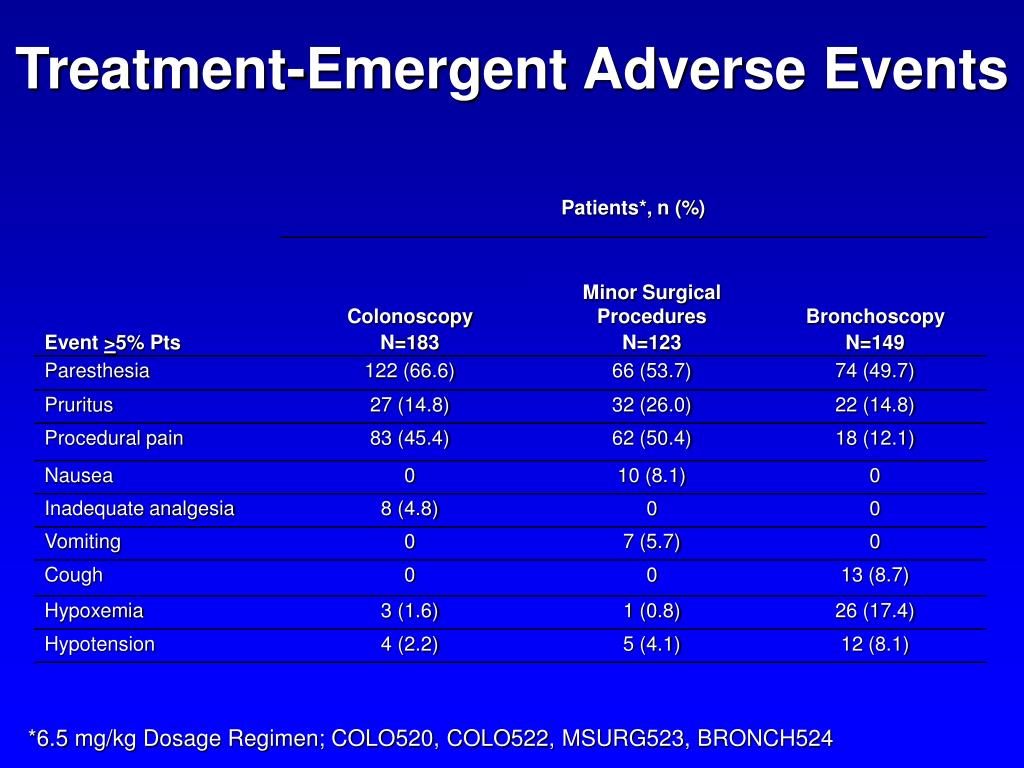
Treatmentemergent adverse events experienced by at least 5 of... Download Scientific Diagram

4567 Background: The randomized phase 3 TIVO-3 study met the primary endpoint of improved PFS with tivozanib (TIVO) vs sorafenib (SOR) in patients with relapsed/refractory mRCC with fewer dose reductions, interruptions and discontinuations despite a longer time on therapy. Greater insight into temporal characteristics of treatment-emergent adverse events (TEAEs) may enable proactive supportive.
Most Common TreatmentEmergent Adverse Event Occurrences (Q3 in Any... Download Table

As the safety profile of this new addition to the mCRPC treatment landscape may be unfamiliar to clinicians and patients, we summarize the data from the literature and provide practical guidelines for the management of treatment-emergent adverse events (TEAEs) that may occur during rucaparib treatment.
Treatmentemergent adverse events Grade 3 and 4 by SOC and preferred term. Download Scientific

Defining Treatment-Emergent Adverse Events with MedDRA 1291 vary from study to study. If it is decided to exclude such patients, then the denomi nator should represent not the number of patients in the treatment group, but rather, the number of patients at risk for TESS event. This issue is not relevant if the
Summary of treatmentemergent adverse events. Download Table

Treatment Emergent Adverse Event, TEAE, defines as "an event that emerges during treatment, having been absent pretreatment, or worsens relative to the pretreatment state" according to the E9 guideline. In crossover clinical trials, TEAE can be more complicated due to several factors such as occurrence in washout period, severity change and.
TreatmentEmergent Adverse Event Download Scientific Diagram

Definitions and Standards for Expedited Reporting). 1.2. Adverse Event (AE) Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment.
PPT Preventing Medical Errors A Team Approach PowerPoint Presentation ID1792249

Treatment-emergent adverse events (TEAEs) that occur close to treatment initiation may negatively affect overall tolerability and adherence. It is important to develop a clear understanding of potential early TEAEs after initiating treatment with cladribine.
Treatmentemergent Adverse Events Download Table

Treatment Emergent Adverse Event, TEAE, defines as "an event that emerges during treatment, having been absent pretreatment, or worsens relative to the pretreatment state" according to the E9 guideline. In crossover clinical trials, TEAE can be more complicated due to several factors such as occurrence in washout period, severity change and.
Treatmentemergent adverse events in patients with chronic... Download Scientific Diagram

• Treatment Emergent Adverse Event An AE for which the start date is on or after the date that the intervention began. • Serious Adverse Events SAEs are a subset of adverse events. An SAE is defined as any untoward medical occurrence that meets any of the following criteria: results in death
Study design. TEAE, treatmentemergent adverse event. Download Scientific Diagram

Treatment-emergent adverse events (TEAE) tables are routinely produced in clinical trials. TEAEs are the events that . occur after treatment or worsen in toxicity after administration of study treatment. The current data collection practices for . adverse events, particularly collection of toxicity changes to an adverse event do vary across the.
Treatmentemergent adverse events per 100 patientyears by years of... Download Scientific Diagram

ABSTRACT. Treatment emergent adverse event (TEAE) tables are mandatory in each clinical trial summary. An adverse event (AE) is counted as treatment emergent in any case when it starts or gets worse during a treatment period (ICH E9). At first glance, it looks simple to detect AEs as treatment emergent.
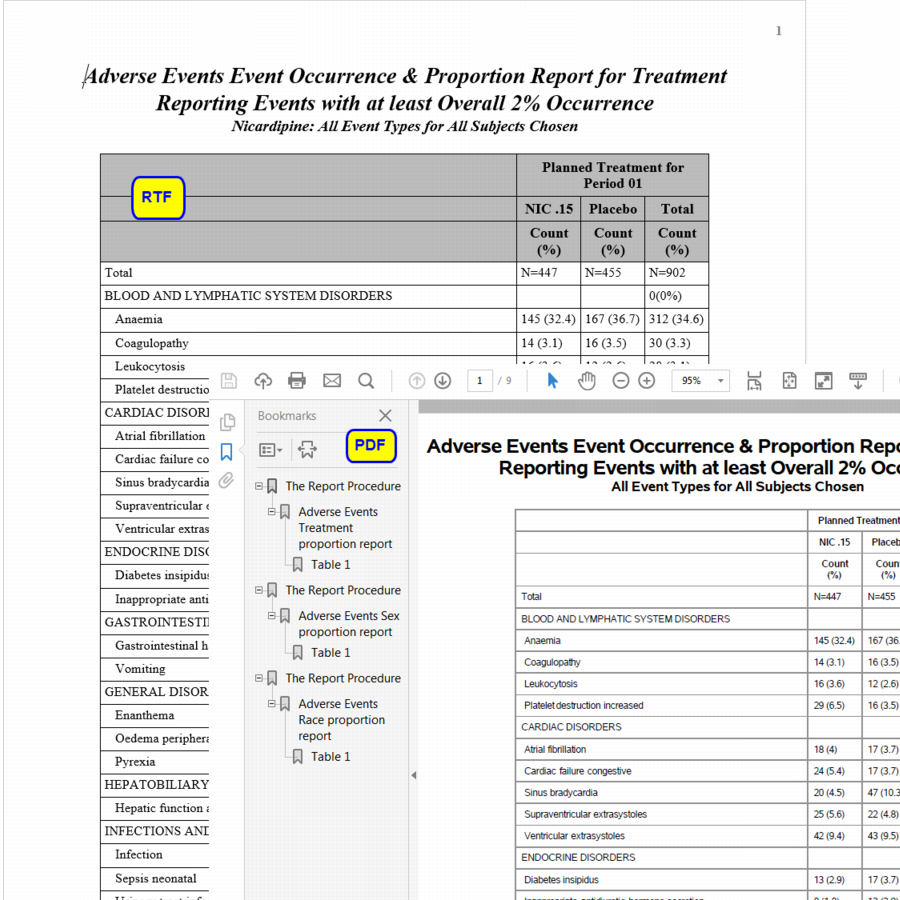
Adverse Events Report
Most prior work focused on serious adverse events and/or those with a GI basis [23-25]. For example, in a population-based, matched cohort study of over 50,000 Medicare patients who underwent outpatient colonoscopy, the overall rate of serious gastrointestinal and cardiovascular adverse events was 0.69 and 1.9 %, respectively . However, the.
Treatmentemergent adverse events (TEAEs) and treatment related* TEAEs... Download Scientific

A response to a medicinal product which is noxious and unintended [DIR 2001/83/EC Art 1(11)]1. Response in this context means that a causal relationship between a medicinal product and an adverse event is at. least a reasonable possibility (see GVP Annex IV, ICH-E2A Guideline). An adverse reaction, in contrast to an.
Treatmentemergent adverse events (SS) Download Scientific Diagram

Summaries of safety data collected in a clinical trial typically include an analysis of the crude rate of treatment-emergent signs and symptoms. This paper outlines the issues in defining treatment-emergent events and discusses the impact of choosing different methodologies for event classification and data collection.
Overview of treatment emergent adverse events (TEAEs). Download Scientific Diagram

Treatment emergent adverse events are undesirable events not present prior to medical treatment, or an already present event that worsens either in intensity or frequency following the treatment.. In medicines development terminology, an adverse event is any undesirable event that occurs after a participant officially consents to take part in a trial (and could occur before treatment begins).
Treatmentemergent adverse events Download Scientific Diagram

not be limited to the definition and data, but other factors like study design, explanation in SAP, derivation rules for partial dates should be kept in consideration.. (E9) a treatment-emergent adverse event is defined as an event that emerges during treatment having been absent pre-treatment, or worsens relative to the pre-treatment state..